Global Stem Cells Group Partners with StemBio to Advance Regenerative Medicine Research and Development
Miami, Florida— Global Stem Cells Group, a leader in regenerative medicine, is thrilled to announce a strategic partnership with StemBio, a renowned leader in the regenerative medicine landscape in Turkey. This partnership aims to propel research and development, conduct clinical trials, and create new products and protocols for the advancement of regenerative medicine.
StemBio, a trailblazer in Turkey, possesses all the necessary licenses and state-of-the-art infrastructure for regenerative medicine, including Bone Marrow Derived MSC, Adipose Derived MSC, Umbilical cord, placenta, Wharton jelly Derived MSC, Synovial Derived MSC, and more. We believe this diverse range of resources demonstrates StemBio’s commitment to excellence, making them one of the foremost facilities for regenerative medicine in Turkey.
This collaboration brings together two powerhouses in the regenerative medicine arena, aiming to break new ground in the research, development, and application of regenerative therapies. We believe the collective expertise and resources of StemBio and Global Stem Cells Group are set to drive innovation and advancements in regenerative care, benefiting patients worldwide.
“We are excited to partner with StemBio and leverage their expertise in regenerative medicine. This partnership should accelerate our mission to provide cutting-edge regenerative solutions to a global audience,” said Benito Novas, head of relationships at Global Stem Cells Group.
We believe the partnership between StemBio and Global Stem Cells Group signifies a significant milestone in the regenerative medicine field. As they combine their strengths and knowledge, they are committed to pushing the boundaries of what is possible in regenerative care and making a lasting impact on patients’ lives.
About Global Stem Cells Group:
The Global Stem Cell Group is a family of several companies focused on stem cell medicine and research. The company uses its network to bring leadership in regenerative medicine training, research, and patient applications.
GSCG’s mission is to allow physicians to present the benefits of stem cell medicine to patients worldwide. The company also partners with policymakers, educators, and regulators to promote regenerative medicine.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website www.stemcellsgroup.com or call +1 305 560 5331
About StemBio:
StemBio is a renowned regenerative medicine facility in Turkey, equipped with cutting-edge infrastructure and a commitment to excellence. Holding the necessary licenses for cord blood and tissue banking, as well as advanced engineering solutions, StemBio is a leader in the regenerative medicine landscape.
Safe Harbor Statement: Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in News
Advancements in Hair Transplantation: A Paradigm Shift in Regenerative Medicine
WEDNESDAY, 09 AUGUST 2023 / PUBLISHED IN BLOG
In the realm of regenerative medicine, hair transplantation has experienced remarkable progress in recent years. As part of a multidisciplinary community of physicians and scientists dedicated to advancing the field, it is essential to stay informed about the latest developments in this area. This blog post aims to provide a comprehensive overview of the cutting-edge advancements in hair transplantation, highlighting the convergence of science, technology, and practice.
Tissue Engineering in Hair Transplantation
The integration of tissue engineering principles into hair transplantation has opened new avenues for regenerative medicine. Scientists are actively exploring the use of biomaterials, growth factors, and scaffolds to create an optimal environment for transplanted hair follicles. By nurturing follicular cells, tissue engineering techniques hold immense potential in enhancing transplantation outcomes and promoting tissue regeneration.
Stem Cell-Based Approaches
Stem cell therapy has emerged as a promising strategy within the realm of hair transplantation. Researchers are investigating the utilization of mesenchymal stem cells (MSCs) and adipose-derived stem cells (ADSCs) to stimulate hair follicle regeneration. These unique regenerative cells promote hair growth and significantly enhance the success rate of transplantation procedures. Ongoing research in stem cell-based therapies continues to unveil their potential to revolutionize hair restoration techniques.
Gene Therapy for Hair Loss
Gene therapy is gaining traction as a cutting-edge approach to addressing hair loss at its core. Scientists aim to develop innovative treatments for both genetic and acquired forms of hair loss by manipulating genes involved in hair growth and regulation. Techniques such as CRISPR-Cas9 gene editing hold immense potential for altering gene expression associated with hair follicle development, offering personalized regenerative therapies.
Nanotechnology in Hair Transplantation
Nanotechnology has paved the way for groundbreaking advancements in hair transplantation. Researchers are exploring the application of nanomaterials and nanoparticles to enhance the targeted delivery of growth factors, drugs, and stem cells directly to the hair follicles. This precise and controlled release optimizes the regenerative potential of transplanted hair follicles, leading to improved treatment outcomes and faster healing.
Artificial Intelligence (AI) and Machine Learning
The integration of AI and machine learning algorithms has transformed various medical fields, including hair transplantation. AI-powered technologies assist in precise hair follicle extraction, optimizing graft placement, and predicting post-transplant outcomes. By analyzing vast datasets and patterns, AI algorithms enhance decision-making, improve surgical techniques, and contribute to personalized treatment plans in hair transplantation.
As active contributors to the advancement of regenerative medicine, staying informed about the latest developments in hair transplantation is crucial. Tissue engineering, stem cell-based approaches, gene therapy, nanotechnology, and the integration of AI and machine learning are reshaping the landscape of hair restoration. By remaining at the forefront of these advancements, physicians and scientists can harness the power of regenerative medicine to effectively treat hair loss and alleviate human suffering.
Join us at issca.us and let us continue to collaborate, innovate, and explore the limitless potential of regenerative therapies.
- Published in Blog
International Society for Stem Cell Applications Launches Advanced Turkish Hair Transplant Certification Program in Cancun
Miami, Florida – The International Society for Stem Cell Applications (ISSCA) is thrilled to announce the launch of its newest program, the Advanced Turkish Hair Transplant Certification. This pioneering training and certification initiative will take place from December 8th to 10th at the prestigious Cellular Hope Institute Facility in Cancun, Mexico. Physicians are encouraged to register promptly as the program is expected to fill up quickly.
Turkey has earned a global reputation for providing the finest hair transplants worldwide. Some of the hair transplant experts from Turkey who have developed and perfected this procedure are now willing to share their secrets with any physician who seeks to excel in hair restoration procedures in their home country. The demand for hair regrowth, hair restoration, and hair transplant procedures is growing by the day as men and women realize they do not have to suffer from the embarrassment of thinning hair and bare scalps anymore.
The Advanced Turkish Hair Transplant Certification program offers a comprehensive curriculum encompassing cutting-edge hair transplant procedures. Participants will engage in both theoretical and practical training sessions, helping to assist their mastery of this rapidly expanding field. They will have the extraordinary opportunity to practice hair transplant surgeries under the supervision and guidance of world-renowned experts in the field, including Veysel Cabalar, Asli Uslu, and Emere Tuncinar, who bring their wealth of experience and knowledge to the program.
In addition to hair transplant surgery, the program’s curriculum will cover workshops on advanced hair restoration techniques such as exomes, stem cells, and platelet-rich plasma treatments. Participants will learn how to select suitable patients for these different hair restoration methods and gain hands-on experience in preparing, storing, and administering exosomes, stem cells, and platelet-rich plasma to promote hair regrowth. Armed with this expertise, we believe participants will return to their own clinics with confidence, ready to deliver these transformative procedures for the benefit of their patients.
Dr. Salih Yildirim, ISSCA President, expressed his delight in fulfilling the organization’s mission of expanding the reach of new regenerative medicine technologies to doctors and patients worldwide: “The Advanced Turkish Hair Transplant Certification program exemplifies our commitment to advancing the field of regenerative medicine. We are proud to provide doctors with the tools and knowledge to bring the latest hair restoration techniques to their patients.”
Benito Novas, ISSCA Vicepresident , added, “We are excited to bring this groundbreaking Turkish training to Cancun, offering local doctors and physicians across Latin America the opportunity to learn from the best. Our collaboration with the Cellular Hope Institute Facility underscores our dedication to providing exceptional educational experiences that benefit medical professionals and patients alike.”
The program will include theoretical sessions on scalp and hair follicle anatomy, patient evaluation, and surgical preparation for hair transplant procedures. Detailed workshops on graft extraction and implantation techniques will also be conducted, enabling participants to refine their skills and achieve realistic, outstanding results. From patient evaluations to postoperative care protocols, the hands-on surgical sessions will encompass every aspect of the hair transplant process.
The Advanced Turkish Hair Transplant Certification program by ISSCA offers an invaluable opportunity for doctors seeking to excel in the field of hair transplantation. Participants will gain comprehensive knowledge, refine their surgical techniques, and stay abreast of the latest advancements in hair regrowth, hair restoration, and hair transplants.
To learn more about the Advanced Turkish Hair Transplant Certification program and register for this transformative educational experience, please visit the official ISSCA website at www.issca.com.
About ISSCA
The International Society for Stem Cell Applications (ISSCA) is a multidisciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through advances in regenerative medicine. By bringing together worldwide researchers and healthcare practitioners, ISSCA aims to bridge the gap between cutting-edge scientific research and compassionate, effective patient care.
About Global Stem Cells Group
Global Stem Cells Group (GSCG) is a world-renowned leader in regenerative medicine, specializing in innovative solutions that harness the power of stem cells for therapeutic applications. With a global network of offices and an unwavering commitment to excellence, GSCG continues to shape the future of regenerative and restorative medicine through groundbreaking products and advanced treatment protocols.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
Safe Harbor Statement: Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in News
Ozone Therapy: A Promising Alternative Treatment with Potential Benefits
MONDAY, 24 APRIL 2023 / PUBLISHED IN BLOG
Ozone therapy is a promising alternative medical treatment that has gained popularity in recent years. It involves the administration of ozone, a form of oxygen, to treat various medical conditions, ranging from chronic pain to infections. Although the scientific evidence on the effectiveness of ozone therapy remains limited, many people claim to have experienced significant health benefits from this alternative treatment. In this article, we’ll explore the potential benefits and risks of ozone therapy, as well as the latest research on this alternative medical treatment.
What is Ozone Therapy?
Ozone therapy is a safe, non-invasive, and effective alternative medical treatment that has been used for over a century. It involves the administration of ozone, a gas made up of three oxygen atoms, to the body in various forms, such as injections, insufflations, or topical applications. Ozone is a powerful oxidant that can damage cells and tissues, which can have positive or negative effects, depending on the form and amount of ozone used.
One of the key benefits of ozone therapy is its ability to improve blood circulation and oxygen delivery to the tissues, which can help reduce inflammation, support wound healing, and boost immune function. Additionally, ozone therapy is believed to have antimicrobial and antiviral properties, which may help fight infections caused by bacteria, viruses, and fungi.
Potential Benefits of Ozone Therapy
Ozone therapy has been used to treat a wide range of medical conditions, including:
- Chronic Pain: Ozone therapy may help reduce pain and inflammation in conditions such as osteoarthritis, herniated discs, and fibromyalgia.
- Infections: Ozone therapy may help fight infections caused by bacteria, viruses, and fungi, including Lyme disease, hepatitis, and HIV.
- Autoimmune Diseases: Ozone therapy may help reduce inflammation and improve immune function in conditions such as multiple sclerosis, rheumatoid arthritis, and lupus.
- Skin Conditions: Ozone therapy may help treat various skin conditions, including eczema, psoriasis, and acne, by improving blood circulation and oxygenation.
Moreover, ozone therapy is a safe and non-invasive treatment that doesn’t have the side effects commonly associated with traditional medical treatments. It’s also relatively inexpensive and can be administered in a variety of settings, including clinics, hospitals, and private practices.
Latest Research on Ozone Therapy
Although the scientific evidence on the effectiveness and safety of ozone therapy remains limited, some recent studies have suggested that ozone therapy may have potential health benefits in certain conditions.
- Immunomodulatory Properties: A study published in the Cuban journal of immunology describes the immunomodulatory properties of ozone therapy by characterizing the biological effects of ozone on immune system cells, soluble mediators, and other cell types.
- Pain Management: A 2022 review on ozone therapy in pain medicine highlights the high safety of pain treatments with ozone therapy, especially modern medical ozone generators with great precision.
- Diabetic Pain Relief: A 2020 study on systemic ozone therapy in insulin-dependent diabetic patients found that the analgesic and anti-inflammatory properties of ozone make it one of the most efficient therapeutic tools for controlling chronic pain in insulin-dependent diabetic patients.
Conclusion
Ozone therapy is a promising alternative medical treatment that has been used to treat various medical conditions. Many people claim to have experienced significant health benefits from this alternative treatment. Furthermore, ozone therapy is a safe and non-invasive treatment that doesn’t have the side effects commonly associated with traditional medical treatments.
If you’re interested in ozone therapy, make sure to do your research, ask questions, and consider this alternative medical treatment as a potential option for your health concerns. It’s important to stay informed about the latest developments in stem cell therapy. You can learn more about regenerative medicine and stem cells by enrolling in our international certification program at www.issca.us.
- Published in Blog
Personalized Regenerative Medicine: The Future of Regenerative Medicine
MONDAY, 17 APRIL 2023 / PUBLISHED IN BLOG
Regenerative medicine is a rapidly growing field that focuses on repairing or replacing damaged or diseased tissues and organs using stem cells, growth factors, and other advanced therapies. The effectiveness of this approach has been demonstrated in treating a wide range of conditions, including heart disease, diabetes, joint pain, and neurological disorders. One of the major challenges of regenerative medicine, however, is that not every patient responds to the same treatment. This is where personalized regenerative medicine comes into play.
What is Personalized Regenerative Medicine?
Personalized regenerative medicine involves tailoring treatments to meet the unique needs of each patient based on their genetic profile, lifestyle, and medical history. This approach recognizes that no two patients are the same and that a one-size-fits-all approach to regenerative medicine is not always effective.
As part of personalized regenerative medicine, advanced technologies such as genomics, proteomics, and metabolomics are used to analyze a patient’s unique biological profile. Regenerative medicine centers utilize this information to develop personalized treatment plans tailored to their individual needs and conditions, ranging from stem cell therapy to gene therapy.
Why is Personalized Regenerative Medicine the Future of Regenerative Medicine?
There are several reasons why personalized regenerative medicine is the future of regenerative medicine:
More Effective Treatments
By tailoring treatments to individual patients, personalized regenerative medicine has the potential to be much more effective than traditional one-size-fits-all approaches.
Improved Patient Outcomes
Because personalized regenerative medicine takes into account the unique needs and conditions of each patient, it has the potential to improve patient outcomes and reduce the risk of complications.
Lower Healthcare Costs
By providing more effective treatments and reducing the risk of complications, personalized regenerative medicine has the potential to lower healthcare costs in the long run.
Better Understanding of Disease
Personalized regenerative medicine involves analyzing a patient’s unique biological profile, which can help researchers and healthcare providers better understand the underlying mechanisms of disease.
Advancements in Technology
As technology continues to advance, personalized regenerative medicine will become even more precise and effective, leading to even better patient outcomes.
Conclusion
The concept of personalized regenerative medicine is an exciting new approach to regenerative medicine that has the potential to revolutionize the healthcare industry. Individualized regenerative medicine has the potential to be more effective, improve patient outcomes, lower healthcare costs, and provide better understanding of disease by tailoring treatments to the individual patient. As technology continues to advance, we can expect to see even more exciting developments in this field in the years to come.
You can learn more about regenerative medicine and stem cells by enrolling in our international certification program at www.issca.us.
- Published in Blog
Revolutionizing Neurodegenerative Care: The Advancements in Stem Cell Therapy
MONDAY, 13 MARCH 2023 / PUBLISHED IN BLOG
Neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis, are a group of incurable neurological disorders characterized by the chronic progressive loss of different neuronal subtypes. The conditions are debilitating and affect millions of people worldwide. Despite its increasing prevalence among the ever-increasing aging population, little progress has been made in the coincident immense efforts towards development of therapeutic agents. However, recent advancements in stem cell therapy, including stem cell-derived exosomes, neurotrophic factors (NTFs), and their combination, show promise as potential therapeutic agents in neurodegenerative diseases.
The Promise of Stem Cell Therapy
In recent years, stem cells have emerged as one of the most active research therapeutic tools for many diseases. Stem cells are undifferentiated cells that have the ability to develop into pluripotent stem cells and adult stem cells. All stem cells have the potential for continuous self-renewal, high proliferation, and multidirectional differentiation into various cell types to replace degenerated or dead cells. In the context of neurodegenerative diseases, stem cells can be used to replace lost or damaged neurons, provide neuroprotection, and promote neural repair. These properties make stem cells an attractive option for treating neurodegenerative diseases, as they have the potential to slow or even reverse the progression of these conditions.
Advantages of Stem Cell Therapy
One of the key benefits of stem cell therapy for neurodegenerative diseases is that it offers a non-invasive, minimally-invasive, and non-toxic alternative to traditional treatments. Unlike drugs and surgery, stem cell therapy does not produce adverse side effects and can be administered in a safe and controlled manner. In clinical trials, stem cell therapy has been shown to be effective in reducing symptoms and improving quality of life for patients with neurodegenerative diseases.
Alzheimer’s Disease (AD)
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and the most common form of dementia, affecting approximately 55 million people worldwide. Cognitive impairment is a hallmark of AD. Currently, drug therapies can only delay symptoms, but not relieve disease pathology or progression. Researchers have demonstrated that neurons derived from stem cells can integrate with existing neural networks and repair damaged neurons within the host brain, improving learning and memory deficits. NTFs can improve AD symptoms and provide neuroprotective effects.
Parkinson’s Disease (PD)
The second most common neurodegenerative disorder is Parkinson’s disease (PD). The motor symptoms of PD mainly include rest tremor, rigidity, bradykinesia, and postural instability, while common nonmotor symptoms include neuropsychiatric and sleep disorders as well as sensory and autonomic dysfunction. At present, there is no cure for PD or disease-modifying therapy. It is common for symptom-relief medications to only provide partial relief and elicit side effects such as motor complications, gastrointestinal problems, and neurological issues. In spite of the fact that these treatments do not address the underlying pathology, alternative therapies, especially those based on stem cells and NTFs, are still being pursued intensively.
Huntington’s Disease (HD)
Huntington’s disease (HD) is characterized by motor, cognitive, and psychiatric dysfunctions. The study of multiple possible neurodegenerative mechanisms of HD is currently underway, and this knowledge is expected to contribute to the development of new HD treatments. The abilities of stem cells to rescue or replace the damaged and dying neurons, and to prevent further cell damage and death, make stem cell-based therapies promising for treatment of this neurodegenerative disease.
Amyotrophic Lateral Sclerosis (ALS)
As a neurodegenerative disorder, amyotrophic lateral sclerosis (ALS) involves progressive degeneration of both upper and lower motor
neurons, resulting in palsy and death within 3-5 years of onset. Currently, only two disease-modifying medicines are available, each showing benefit to a limited number of patients. Stem cell-based therapy holds great promise for treating ALS by providing both cell replacement and NTF delivery to target the multiple pathologies. Stem cells available for ALS treatment include NSCs, MSCs, embryonic stem cells, induced pluripotent stem cells, and olfactory ensheathing stem cells.
The Future of Stem Cell Therapy in Neurodegenerative Diseases
While stem cell therapy is still in the early stages of development, it holds enormous promise as a future treatment for neurodegenerative diseases. A number of studies have demonstrated that stem cells and NTFs offer considerable therapeutic potential, especially when they are used together. In addition to replenishing target neurons, stem cells combined with NTFs can produce neurotrophins to improve the microenvironment that promotes nerve repair and regeneration. As research continues, we can expect to see even more exciting advancements in this field, bringing hope to those affected by these debilitating conditions.
Conclusion
Stem cell therapy represents a revolutionary approach to treating neurodegenerative diseases, offering a non-toxic, minimally-invasive alternative to traditional treatments. With ongoing research and development, the potential to improve patient outcomes and quality of life is immense. It’s important to stay informed about the latest developments in stem cell therapy. You can learn more about regenerative medicine and stem cells by enrolling in our international certification program at www.issca.us.
- Published in Blog
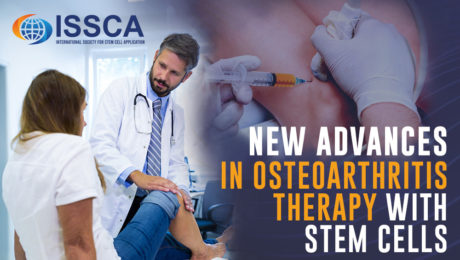
New advances in osteoarthritis therapy with stem cells
FRIDAY, 18 NOVEMBER 2022 / PUBLISHED IN BLOG
Osteoarthritis is a rheumatic pathology that damages the articular cartilage. By joining two bones through the joint capsule, the joints are able to move, providing us with functional autonomy. An inner fluid called synovial fluid is usually found within joints, which is produced by the synovial membrane. Articular cartilage covers the ends of the bones that form the joint. As a result of damage to this articular cartilage, pain, stiffness, and functional impairment occur. Osteoarthritis is the most common joint disorder, usually beginning between the ages of 40 and 50, affecting to some degree almost everyone over the age of 80. Typically, osteoarthritis affects the spine, shoulders, fingers, hips, knees, and toe joints.
Potential of Stem Cell Therapies
Stem cell therapies have the potential to treat a broad spectrum of diseases, including osteoarthritis, rhizarthrosis, diabetes, neurodegenerative diseases, spinal cord injuries, and heart disease. By utilizing stem cells, regenerative medicine is capable of repairing tissues in affected areas. The main difference between lipogem therapy and other treatments for osteoarthritis is that lipogem therapy regenerates cartilage, avoids surgery and its sequelae, and improves the quality of life for patients.
Advances in Sports Medicine and Traumatology
The potential for medical treatments with stem cells and their by-products is currently very high. In the field of sports medicine and traumatology, one of the most outstanding advances has been made for the first time in decades recently: Spanish scientists have achieved a degree of tendon regeneration in 100% of injured patients, resulting in a decrease in pain and a return to sport within two months, and just six months after the trial was completed.
A research performed by the Institute of Regenerative Tissue Therapy (ITRT), published by the prestigious American Journal of Sports Medicine, demonstrates how this therapy regenerates chronic lesions in the patellar tendon and opens up a new therapeutic option for this tissue, which was considered impossible to regenerate.
Adipose Tissue-Derived MSCs
In most patients, fat tissue can be harvested minimally invasively (under local or general anesthesia), providing a highly viable MSC population regardless of donor age. Similar to MSCs derived from other tissues, adipose tissue-derived MSCs have regenerative potential. As osteoarthritis is a very common joint disease, and knee osteoarthritis is the most common form, it is necessary to review scientific literature on osteoarthritis treatments with stem cells, like lipogems.
Lipogems Therapy
Lipogems therapy is a novel procedure that enhances the body’s natural ability to heal itself through the innovative power of science and biotechnology. The Lipogems method involves injecting mesenchymal stem cells into the joints. Adipose-derived mesenchymal stem cells have enormous regenerative potential. They also have a regenerative capacity independent of their age. Even older individuals can benefit from this procedure.
Injection of mesenchymal stem cells into the knee, particularly in the early stages of osteoarthritis, can stop the process of inflammation and degeneration, especially in the less advanced stages of the disease. In addition to preventing progressive physical deterioration of the articular cartilage, this treatment contributes significantly to a patient’s well-being and prevents the installation of knee prostheses.
Patellar Tendinopathy: Physiotherapeutic Treatment and Stem Cell Therapy
Injuries to the patellar tendon that connects the kneecap to the tibia are known as patellar tendinopathy or patellar tendinitis. The patellar tendon works with the muscles in the front of the thigh to extend the knee so you can kick, run, and jump. Athletes who perform frequent jumping in their sports, such as basketball and volleyball, are most likely to suffer from patellar tendonitis. However, people who don’t engage in jumping sports may develop patellar tendonitis. Patients with patellar tendinitis usually begin treatment with physical therapy to stretch and strengthen their knee muscles.
Strength Training and Stem Cell Therapy for Tendinopathies
Strength training with eccentric resistance is one of the most common treatments for tendinopathies. Alternatively, it has been demonstrated that bone marrow-derived mesenchymal stem cells (MSCs) can regenerate injured patellar tendons. Within six months of treatment, it has been observed that the structure of this tissue – which is always difficult to treat – is restored, reaching a regeneration of 40% in all injured persons, with a gradual improvement that eventually becomes complete.
Combination Therapies for Patellar Tendinopathy
It has been found that traditional management methods, including isometric or eccentric exercises, shock wave therapy, and even surgery, are not effective. As part of a rehabilitation program in chronic patellar tendinopathy, autologous expanded bone marrow mesenchymal stem cells (BM-MSC) or leukocyte-poor platelet-rich plasma (Lp-PRP) may be effective in reducing pain and improving activity levels. Traditional management, which includes isometric or eccentric exercises, shock wave therapy, and even surgery, has limited success. A combination of autologous expanded bone marrow mesenchymal stem cells (BM-MSCs) and leukocyte-poor platelet-rich plasma (Lp-PRP) and rehabilitation may reduce pain and improve activity levels in active participants with chronic patellar tendinopathy.
To learn more about stem cells, cellular therapies, and keep up to date with all the information about regenerative medicine and its advances, sign up for our international certification in regenerative medicine at www.issca.us.
- Published in Blog
Regenerative Medicine – An Overview On Stem Cell Therapy
THURSDAY, 10 NOVEMBER 2022 / PUBLISHED IN BLOG
Stem cell therapy is a form of regenerative medicine designed to repair damaged cells within the body by reducing inflammation and modulating the immune system. This phenomenon makes stem cell therapy a viable treatment option for a variety of medical conditions.
What is Stem Cell Therapy?
The term stem cell therapy refers to any treatment involving the use of viable human stem cells, including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells. Stem cells have the unique ability to differentiate into specific cell types necessary for repairing diseased tissues, making them ideal for tissue and organ transplantation.
The Hierarchy of Stem Cells
In general, stem cells fall into three categories based on their differentiation potential:
- Totipotent Cells: The most basic and capable of forming a complete embryo and extraembryonic tissues.
- Pluripotent Cells (Embryonic Stem Cells): Can differentiate into any of the three embryonic germ layers (ectoderm, mesoderm, and endoderm).
- Adult Stem Cells: Found in specialized tissues throughout the body, capable of differentiating into specific cell types related to their tissue of origin.
Adult stem cells are considered the gold standard for stem cell-based therapies due to their versatility and ability to maintain homeostasis throughout an organism’s lifespan.
Stem Cell Research for Treating Disease
Stem cell research has made significant advancements, particularly with induced pluripotent stem cells (iPSCs), which are generated from adult cells and share properties with embryonic stem cells. This innovation has enhanced disease identification and treatment possibilities, particularly in autologous transplants using patient-matched cells.
Applications of Stem Cells
Stem cells hold potential for treating diseases such as diabetes and heart disease due to their regenerative capabilities. Ongoing research aims to better understand how stem cells can be utilized in regenerative and reparative medicine to effectively treat various conditions.
Future Directions in Stem Cell Research
Stem cell studies continue to expand our knowledge of cell development and regeneration. Researchers are leveraging stem cells to test new drugs, develop model systems for studying normal development, and investigate the causes of birth defects.
To learn more about stem cells, cellular therapies, and new medical protocols using exosomes, sign up for our international certification in regenerative medicine at www.issca.us.
- Published in Blog
Global Stem Cells Group Announces the Opening of a Multi-Specialty Regenerative Medicine Center in Cancun
Global Stem Cells Group has just announced the official opening of a new multi-specialty regenerative medicine center in Cancun. The new center is intended to incorporate different treatments based on regenerative medicine as well as to serve as a multi-specialty training center in the field of regenerative medicine and cellular therapies, offering doctors and patients the most advanced treatments in cell therapy worldwide.
The Cellular Hope Institute, as this facility was named, will also strive to pursue several objectives that will benefit patients and medical experts worldwide. It is expected to be a medical hub that will offer the best treatments for patients suffering from multiple conditions such as autoimmune deficiencies, sports injuries, chronic degenerative diseases and pediatric diseases.
Benito Novas says. “Our goal is to solve the problem and alleviate the condition, not just attempt to treat it. We are committed to offering top-notch technology available and quality care, emphasizing that our patients are always our number one priority. Our highly qualified team of experts are always ready to assist you at Cellular Hope Institute.”
The Center utilizes comprehensive stem cell treatment protocols that employ well-targeted combinations of Exosomes, allogeneic human Mesenchymal cells, autologous bone marrow and Adipose derived stem cells to treat the diseases and conditions listed above. The treatment plans are mostly focused on a systemic or whole-body approach to ensure patients receive the highest quality and quantity of cellular products available today.
Cellular Hope Institute will also be a center that will offer specialized regenerative medicine courses for physicians. Primarily, it is expected to be a resource center that will train doctors to incorporate modern regenerative medicine practices into their offices.
Our focus is to incorporate multiple specialties into separate programs within this training hub. Industry experts are expected to join the facility and offer the latest information about various compounds in the stem cell market, such as Mesenchymal stem cells, umbilical cord derived products, exosomes and many other stem cell products. We plan to target physicians who want to incorporate the treatment solutions into their practices, and the separate programs are designed to reach a wide array of practitioners.
In addition, Cellular Hope Institute also expects to have a fully functional manufacturing laboratory that seeks to elaborate the cellular products. The laboratory is already equipped with the latest and innovative technology to ensure it offers the best stem cell products and treatments. The facility will have enough capacity to store and distribute cellular products, including cells and exosomes. We expect it to be the primary supply center for doctors all over Mexico. The inauguration will mark a step closer to pursuing cell therapy, regenerative medicine, and modern tissue engineering options globally.
The opening of this center is an effort by Global stem Cells Group to continue its mission to reduce the suffering of patients and offer a new healing alternative. It is the commitment of scientists and doctors who believe in the potential of regenerative medicine as a standard treatment that should be in every doctor’s office worldwide.
To learn more about the cellular hope institute, visit its website here: https://cellularhopeinstitute.com/
- Published in News