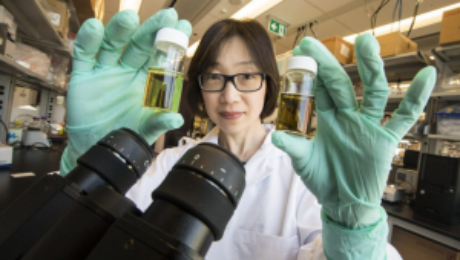
Stem Cell “Tattoo” Technology Allows Researchers to Track Cell Implants Non-invasively
TUESDAY, 12 JULY 2016 / PUBLISHED IN BLOG
Introduction to Stem Cell Tattoo Technology
Researchers at the University of Toronto have developed a tracer ink—a “stem cell tattoo”—that provides the ability to monitor stem cells in unprecedented detail after they’re injected. The research findings, titled “Bifunctional Magnetic Silica Nanoparticles for Highly Efficient Human Stem Cell Labeling,” were published in June in the Journal of Magnetic Resonance Imaging. Already emerging as an ideal probe for noninvasive cell tracking, the technology has the potential to revolutionize stem cell research by arming scientists with the ability to visually follow the pathways and effectiveness of stem cell therapies in the body in real time.
Advancements in Stem Cell Tracking
University of Toronto biomedical engineering professor Hai-Ling Margaret Cheng, a biomedical engineer who specializes in medical imaging, says the new technology allows researchers to actually see and track stem cells after they’re injected. Cheng hopes the technique will help expedite the development and use of stem cell therapies.
Development of the Contrast Agent
Working with colleague Xiao-an Zhang, an assistant professor of chemistry at the University of Toronto, Scarborough, Cheng developed a singular chemical compound known as a contrast agent that acts as a tracer. Composed of manganese, an element that naturally occurs in the body, this tracer compound, called MnAMP, bathes stem cells in a green solution, rendering them traceable inside the body under MRI.
Long-term Cell Tracking with Tracer Ink
The contrast agent “ink” first enters a stem cell by penetrating its membrane. Once inside, it stimulates a chemical reaction that prevents it from seeping out of the cell the same way it entered. Previous versions of contrast agents easily escaped cells. By establishing a way to contain the ink within the cell’s walls, the research team achieved the ability to track the cells long term once they are inside the body.
Advantages of the New Technology
According to Cheng, some basic contrast agents are already available for use in humans, but none are capable of tracking cells over a long period of time. Contrast agents work by illuminating the deepest and darkest corners of a person’s internal architecture so they appear clearly under X-rays, computed tomography (CT) scans, and MRIs. An example of a currently used contrasting agent would be the barium sulfate solution given to patients to help diagnose certain disorders of the esophagus, stomach, or intestines.
Non-invasive Cell Tracking Benefits
Before the stem cell tattoo tracer ink was developed, surgery was the only option for scientists to get a literal glance of a cell’s destiny after it was injected into the body. Now, researchers can track the results in real time without resorting to any invasive procedures. “Before, we could not visually track the cells once they were introduced into the body,” Cheng says. “Now we have the ability to view cells in a non-invasive manner using MRI, and monitor them for potentially a very long time.”
Current Stage of Development

Currently, the tracer ink technology is still in the early development phase and requires more animal testing. Cheng is hopeful it can proceed to human clinical trials in about 10 years. While Cheng has already proven that tattooing an animal’s embryonic stem cell doesn’t affect its ability to transform into a functional heart cell, larger models, such as rats or pigs (which better represent human size), are up for evaluation next.
Future Testing and Applications
In those test cases, researchers will cut off and reduce blood flow in the animals to mimic the effects of damage caused by a human heart attack. Cardiac stem cells pre-tagged with Cheng’s ink tracer technology will then be injected into the damaged tissue. Using MRI to monitor the luminous inked stem cells in action, researchers can non-invasively follow where in the body they’re traveling and more easily determine if the new cells are responsible for restoring normal heart rhythm. Before it can be tested in humans, the chemical tracer will also have to pass rigorous toxicology tests to ensure its safety.
- Published in Blog
Stem Cell-stimulating Fillings Help Regenerate Teeth Damaged by Disease, Decay
MONDAY, 11 JULY 2016 / PUBLISHED IN BLOG
Introduction to Stem Cell-stimulating Fillings
Researchers from Harvard University and the University of Nottingham have developed a new filling that stimulates stem cells in dental pulp to regenerate and even regrow teeth damaged by disease and decay. According to Newsweek Magazine, the discovery earned a prize from the Royal Society of Chemistry after judges described it as a “new paradigm for dental treatments.” The treatment is believed to potentially eliminate the need for root canals.
How the Filling Material Works
The filling works by stimulating the body’s natural store of stem cells to encourage the growth of dentin—the bony material that makes up the majority of the tooth—allowing patients to effectively regrow teeth that are damaged through dental disease. The filling’s synthetic biomaterials are used similarly to dental fillings, placed in direct contact with pulp tissue in the damaged tooth. This stimulates the tissue’s native stem cell population to repair and regenerate pulp tissue and the surrounding dentin.
Comparison to Current Dental Treatments
The discovery is a significant step forward from current methods to treat cavities, which involve drilling out decay and putting in a filling made of gold; porcelain; silver amalgam (which consists of mercury mixed with silver, tin, zinc, and copper); or tooth-colored plastic or composite resin. When these fillings fail to halt the tooth’s decay, a root canal is needed to remove the pulp of the tooth, damaging it even further.
Potential for Industry Adoption
Researchers hope to develop the technique with industry partners to make it available for dental patients as an alternative to traditional fillings. Marie Curie research fellow Adam Celiz says that existing dental fillings are toxic to cells and are therefore incompatible with pulp tissue inside the tooth. “In cases of dental pulp disease and injury, a root canal is typically performed to remove the infected tissues,” Celiz says.
Recognition and Awards
The promise of using therapeutic biomaterials to bring stem cell medicine to restorative dentistry could significantly impact millions of dental patients each year. The approach is so promising it won second prize in the materials category of the Royal Society of Chemistry’s Emerging Technology Competition for 2016. Competition entries were judged on the degree of innovation of the technology, its potential impact, and the quality of the science behind it.
Future of Dentistry with Stem Cell Technology
The stem cell-stimulating filling promises to change the future of dentistry, according to David Mooney, Pinkas Family Professor of Bioengineering at the John Paulson School of Engineering and Applied Sciences at Harvard and the Wyss Institute for Biologically Inspired Engineering. “These materials may provide an effective and practical approach to allow a patient to regenerate components of their own teeth,” Mooney says.
Broader Implications of Stem Cell Research
Stem cells can induce regenerative, self-healing qualities in any tissue found in the body and can, as a result, provide unlimited potential for medical applications. Current studies are underway worldwide to learn how stem cells may be used to prevent or cure diseases and injuries such as Parkinson’s disease, type 1 diabetes, heart disease, spinal cord injury, muscular dystrophy, Alzheimer’s disease, strokes, burns, osteoarthritis, vision and hearing loss, and more. Stem cells may also be used to replace or repair tissue damaged by disease or injury
- Published in Blog
What are the potential uses of human stem cells? What obstacles must still be overcome before these potential uses will be realized?
TUESDAY, 28 JUNE 2016 / PUBLISHED IN BLOG
Introduction to Human Stem Cells and Their Potential Uses
There are many ways in which human stem cells can be used in research and in the clinic. Studies of stem cells continue to yield information about their complex capabilities. A primary goal of this research is to identify how undifferentiated stem cells become the differentiated cells that form the tissues and organs. Scientists know that turning genes on and off is central to this process. Some of the most serious medical conditions, such as cancer and birth defects, are due to abnormal cell division and differentiation.
Understanding Genetic and Molecular Triggers
A more complete understanding of the genetic and molecular triggers of these conditions can yield information about how they arise and suggest new strategies to treat them. Predictably controlling cell proliferation and differentiation requires additional basic research on the molecular and genetic signals that regulate cell division and specialization. While recent developments with induced pluripotent stem cells (iPSCs) suggest some of the specific factors that may be involved, techniques must be developed to introduce these factors safely into the cells and control the processes that are induced by these factors.
Human Stem Cells and Drug Testing
Human stem cells are also being used to test new drugs. New medications are tested for safety on differentiated cells generated from human pluripotent cell lines. Other kinds of cell lines have a long history of being used in this way. Cancer cell lines, for example, are used to screen potential anti-tumor drugs. The availability of pluripotent stem cells would allow drug testing on a wider range of cell types. However, to screen drugs effectively, the conditions must be identical when comparing different drugs. Therefore, scientists must be able to precisely control the differentiation of stem cells into the specific cell type on which drugs will be tested.
Challenges in Drug Testing with Stem Cells
For some cell types and tissues, current knowledge of the signals controlling differentiation falls short of being able to mimic these conditions precisely to generate pure populations of differentiated cells for each drug being tested.
Human Stem Cells in Cell and Tissue Generation

Perhaps the most important potential application of human stem cells is the generation of cells and tissues that could be used for cell-based therapies. Today, donated organs and tissues are often used to replace ailing or destroyed tissue, but the need for transplantable tissues and organs far outweighs the available supply. Stem cells, directed to differentiate into specific cell types, offer the possibility of a renewable source of replacement cells and tissues to treat diseases including macular degeneration, spinal cord injury, stroke, burns, heart disease, diabetes, osteoarthritis, and rheumatoid arthritis.
- Published in Blog
Mending a Broken Heart and Addressing Diabetes with Adult Stem Cells
TUESDAY, 28 JUNE 2016 / PUBLISHED IN BLOG
Introduction to Stem Cell Research for Heart Disease and Diabetes
Researchers are learning about mending a broken heart–that is, how to generate healthy heart muscle stem cells in the laboratory and then transplant those cells into patients with chronic heart disease. Preliminary research in mice and other animals indicates that bone marrow stromal cells, transplanted into a damaged heart, can have beneficial effects. Whether these cells can generate heart muscle cells or stimulate the growth of new blood vessels that repopulate the heart tissue, or help through some other mechanism is actively under investigation.
Stem Cell Research: Mending a Broken Heart
For example, injected cells may accomplish repair by secreting growth factors, rather than actually incorporating into the heart. Promising results from animal studies have served as the basis for a small number of exploratory studies in humans. Other recent studies in cell culture systems indicate that it may be possible to direct the differentiation of adult bone marrow cells into heart muscle cells.
Can Stem Cells Mend a Broken Heart?
For that matter, what can stem cells do to treat all cardiovascular diseases including hypertension, coronary heart disease, stroke, and congestive heart failure? Cardiovascular disease (CVD) has ranked the number one cause of death in the United States every year since 1900 except 1918, when the nation struggled with an influenza epidemic. Nearly 2,600 Americans die of CVD each day—roughly one person every 34 seconds. Given the country’s large aging population and the relatively dramatic recent increases in the prevalence of cardiovascular risk factors such as obesity and type 2 diabetes, CVD will be a significant health concern for decades to come.
Strategies to Treat Heart Disease with Stem Cells
Cardiovascular disease can deprive heart tissue of oxygen, thereby killing cardiac muscle cells (cardiomyocytes). This loss triggers a cascade of detrimental events, including formation of scar tissue, an overload of blood flow and pressure capacity, the overstretching of viable cardiac cells attempting to sustain cardiac output leading to heart failure, and eventual death. Restoring damaged heart muscle tissue, through repair or regeneration, is therefore a potentially new strategy to treat heart failure.
Research on Adult-Derived Stem Cells for Cardiac Repair
The use of adult-derived stem cells for cardiac repair is an active area of research. A number of stem cell types, including cardiac stem cells that naturally reside within the heart, myoblasts (muscle stem cells), adult bone marrow-derived cells including mesenchymal cells (bone marrow-derived cells that give rise to tissues such as muscle, bone, tendons, ligaments, and adipose tissue), endothelial progenitor cells (cells that give rise to the endothelium, the interior lining of blood vessels), and umbilical cord blood cells, have been investigated as possible sources for regenerating damaged heart tissue. All have been explored in mouse or rat models, and some have been tested in larger animal models, such as pigs.
Human Studies on Cardiac Repair with Stem Cells
A few small studies have also been carried out in humans, usually in patients who are undergoing open-heart surgery. Several of these have demonstrated that stem cells that are injected into the circulation or directly into the injured heart tissue appear to improve cardiac function and/or induce the formation of new capillaries. The mechanism for this repair remains controversial, and the stem cells likely regenerate heart tissue through several pathways. However, the stem cell populations that have been tested in these experiments vary widely, as do the conditions of their purification and application. Although much more research is needed to assess the safety and improve the efficacy of this approach, these preliminary clinical experiments show how stem cells may one day be used to repair damaged heart tissue, thereby reducing the burden of cardiovascular disease.
Treating Type I Diabetes with Stem Cells
In people who suffer from type 1 diabetes, the cells of the pancreas that normally produce insulin are destroyed by the patient’s own immune system. New studies indicate that it may be possible to direct the differentiation of human embryonic stem cells in cell culture to form insulin-producing cells that eventually could be used in transplantation therapy for persons with diabetes.
Steps for Successful Cell-Based Treatments
To realize the promise of novel cell-based therapies for such pervasive and debilitating diseases, scientists must be able to manipulate stem cells so that they possess the necessary characteristics for successful differentiation, transplantation, and engraftment. The following is a list of steps in successful cell-based treatments that scientists will have to learn to control to bring such treatments to the clinic. To be useful for transplant purposes, stem cells must be reproducibly made to:
- Reproduce extensively and generate sufficient quantities of cells for making tissue.
- Differentiate into the desired cell type(s).
- Survive in the recipient after transplant.
- Integrate into the surrounding tissue after transplant.
- Function appropriately for the duration of the recipient’s life.
- Avoid harming the recipient in any way.
Avoiding Immune Rejection in Stem Cell Therapies
Also, to avoid the problem of immune rejection, scientists are achieving good results with strategies that use the patient’s own stem cells to generate tissue that will not be rejected.
- Published in Blog
Stem Cell Myths, Busted
THURSDAY, 02 JUNE 2016 / PUBLISHED IN BLOG
Introduction to Stem Cell Myths and Facts
The term stem cell research gleans different reactions from people, both in the medical community and the wider public. Still an emerging science, stem cell research is shrouded by many myths and misconceptions. Here, we take on some of the most predominant myths to discuss the misconceptions and clarify the facts regarding this fast-growing branch of medicine.
Myth #1: Stem Cells Only Come from Embryos
FACT: False. Stem Cells Exist in All Bodies, from Embryos to Adults
Embryonic stem cells come from the early embryo and have the potential to produce all the specialized cells of the body. Because of this, they hold great promise for studying and potentially treating disease and injuries. Tissue or “adult” stem cells are found in the body throughout our lives. These cells maintain and repair many tissues in the body. Examples of these cells include blood stem cells, muscle stem cells, bone marrow stem cells, adipose tissue (fat) stem cells, and skin stem cells. Some of these adult stem cells are used in established medical and aesthetic treatments.
Myth #2: Induced Pluripotent Stem Cells (iPSCs) Eliminate the Need for Embryonic Cells
FACT: False. Research is Needed on All Types of Cells
It is not clear which cells will be most useful for which types of application. For the foreseeable future, side-by-side research on both embryonic and induced pluripotent stem cells is needed. Global Stem Cell Group’s research and treatment products use no embryonic stem cells.
Myth #3: Stem Cell Research Leads to Cloning Humans
FACT: False. Most Countries Prohibit Human Cloning

In most countries, even attempting to clone a human being is illegal. Some countries do allow something called “therapeutic cloning” for the purposes of studying a disease. In this procedure, scientists isolate embryonic stem cells from a cloned blastocyst (early stage embryo) but do not transfer the blastocyst into a womb. These stem cells are genetically matched to the donor organism for studying genetic disease. For example, stem cells could be generated using the nuclear transfer process described above, with the donor adult cell coming from a patient with diabetes or Alzheimer’s. The stem cells could be studied in the laboratory to help researchers understand what goes wrong in diseases like these. Therapeutic cloning also could be used to generate cells that are genetically identical to a patient’s. A patient transplanted with these cells would not suffer the problems associated with transplant rejection. To date, no human embryonic stem cell lines have been derived using therapeutic cloning.
Myth #4: Adult Stem Cells Are Only Found in Adults
FACT: False. Tissue Stem Cells Are Found in People of All Ages
There are three different types of stem cells: embryonic stem cells, induced pluripotent stem cells, and tissue-specific stem cells. It’s the tissue stem cells that are often called “adult” stem cells, but these “adult” stem cells are found in people of all ages.
Myth #5: Embryonic Stem Cell Research Is Banned in Europe
FACT: False. The Laws Vary Across the EU
EU member states have diverging regulatory positions on human embryonic stem cell research. For instance, in Germany, the use of embryos for research is heavily restricted under the Embryo Protection Act (Embryonenschutzgesetz) of 1991, which makes the derivation of embryonic stem cell lines a criminal offense. But in the UK, embryonic stem cell research is allowed, subject to licensing from the Human Fertilization and Embryology Authority (HFEA). Click here for country-by-country overviews for more details. Under the previous two European Framework programs (FP6 and F7), as well as the current program, Horizon 2020, human embryonic stem cell research can be funded, provided that the work is permitted by law in the country where it is to take place.
Myth #6: Stem Cell Research and Treatment Is Against the Law in the US
FACT: False. The FDA Regulates Stem Cell Products but They Are Not Illegal

The FDA does not regulate the practice of medicine, but rather drugs and medical devices and which of these can be marketed in the US. Under federal law, cultured (grown) stem cell products are considered a drug, but are not illegal. Adult stem cells, however, are not cultured—they exist in our bodies throughout our organs, blood, skin, teeth, fat, bone marrow, and other places. Adult stem cell therapy is currently used in the United States to treat conditions such as leukemia and other illnesses. Bone marrow consists of stem cells which have been transplanted for years in the US. Global Stem Cells Group offers stem cell treatments in countries where stem cell therapy is approved and regulated with no appreciable difference in safety record. Stem cell therapy technology is still under review by the FDA.
Myth #7: Bone Marrow Is the Best Source of Stem Cells
FACT: False. Bone Marrow Is Just One Source of Stem Cells
Bone marrow stem cells have been studied for decades and have been used to treat certain types of cancer. A great deal of research has been dedicated to understanding this source of stem cells and their potential. Bone marrow contains a number of different kinds of stem cells, one of which is mesenchymal stem cells. However, mesenchymal stem cells can also be found in adipose (fat) tissue at nearly 2000 times the frequency of bone marrow. Mesenchymal cells have the capability to become different types of tissues (blood vessels, muscle tissue, etc.) and are capable of communicating with other cells. In combination with other proteins, molecules, and regenerative cells found in adipose tissue, they also have the ability to reduce inflammation, regenerate damaged tissue, and grow new blood vessels, a process known as angiogenesis. Stem cells from adipose tissue are more accessible and abundant. They can be processed immediately and reintroduced into the body right away.
Myth #8: There Is a Risk of Rejection with Stem Cell Therapy
FACT: False. Autologous Stem Cells Eliminate the Risk of Rejection
When a patient’s stem cells are derived from his or her own body (such as fat tissue), there is no risk of rejection. In fact, studies thus far have indicated no safety issues with fat-derived autologous (from self) stem cells. Since these stem cells come from your own body, the risk of rejection is eliminated.
- Published in Blog
Stem Cell Treatments Normally Used for Cancer Patients are Helping Multiple Sclerosis Patients
MONDAY, 04 APRIL 2016 / PUBLISHED IN BLOG
Introduction
The British Broadcasting Corporation (BBC) recently reported that stem cell transplant treatments normally used for cancer patients are helping Multiple Sclerosis (MS) patients in the UK. According to the January 18, 2016 report, 20 patients received bone marrow stem cell transplants using their own stem cells, and at least some of the patients who were paralyzed by MS are able to walk again post-treatment.
Impact of Multiple Sclerosis in the UK
Approximately 100,000 people in the United Kingdom suffer from MS, with most new patients diagnosed between the ages of 20 and 30 years. “To have a treatment which can potentially reverse disability is really a major achievement,” says Prof Basil Sharrack of Sheffield’s Royal Hallamshire Hospital in Sheffield, England.
Autologous Hematopoietic Stem Cell Transplantation (HSCT)
The treatment, known as autologous hematopoietic stem cell transplantation (HSCT), involves the intravenous infusion of autologous or allogeneic stem cells harvested from the patient’s own bone marrow to reestablish hematopoietic function (formation of blood or blood cells) in patients whose bone marrow or immune system is damaged or defective by chemotherapy. Using stem cells harvested from the patient’s bone marrow helps rebuild the immune system. The theory is that these newly harvested cells are at such an early stage in development that the cellular defects that result in MS do not exist. “The immune system is being reset or rebooted back to a time point before it caused MS,” says Prof John Snowden, consultant hematologist at Royal Hallamshire Hospital.
Patient Success Stories
The BBC’s Panorama program spoke to several MS patients who have undergone the stem cell transplant. Steven Storey was diagnosed with MS in 2013 and, within a year, went from being an able-bodied athlete to wheelchair dependent and losing sensation in much of his body. “I went from running marathons to needing 24-hour acute care. At one point I couldn’t even hold a spoon and feed myself,” Storey says.

Clinical Trials and Research
The Royal Hallamshire Hospital along with hospitals in the United States, Sweden, and Brazil, is part of an international clinical trial called MIST that is assessing the long-term benefits of the stem cell procedure on MS patients. Study participants all have relapsing-remitting MS (RRMS) and received intensive chemotherapy to completely destroy the patients’ immune systems.
Cost and Accessibility of Treatment
Treatment costs are about the same as the annual cost for existing treatments, and the stem cell treatment does not require the use of new or existing medications. Prof Richard Burt of Northwestern University in Chicago carried out the first hematopoietic stem cell transplantation for MS in 1995, and is coordinating this current MIST international trial, which began in 2006. “There has been resistance to this in the pharma and academic world,” Burt says. “This is not a technology you can patent and we have achieved this without industry backing.”
Study Results and Future Implications
A study published last year involving MS patients in Chicago showed significant reductions in neurological disability, and for some, the improvements persisted for at least four years, although there was no comparative control group. The outcomes of the current international trial will be reported in 2018 and may determine whether the stem cell transplant becomes a standard in the United Kingdom’s healthcare system for many MS patients. “Ongoing research suggests stem cell treatments such as HSCT could offer hope, and it’s clear that in the cases highlighted by Panorama they’ve had a life-changing impact,” says Emma Gray, M.D., head of clinical trials at UK’s MS Society.
- Published in Blog
How Stem Cell Therapies Can Help Heal Sports Injuries
MONDAY, 14 MARCH 2016 / PUBLISHED IN BLOG
Introduction to Stem Cell Therapies in Sports Medicine
Continuing our recent discussion of stem cell therapies for sports injuries, the use of mesenchymal stem cells (MSCs) in orthopedic medicine can help in the repair of damaged tissue by harnessing the healing power of undifferentiated cells that form all other cells in our bodies. The process involves isolating these stem cells from a sample of your blood, bone marrow, or adipose tissue (fat cells), and injecting it into the damaged body part to promote healing. Platelet-rich plasma (PRP), a concentrated suspension of platelets (blood cells that cause clotting of blood) and growth factors, is also used to assist the process of repair.
Cartilage Damage

Cartilage has long been considered an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood supply network. Of particular interest to researchers is the repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
Recently, researchers have increased their focus on the use of MSCs for treatment of cartilage damage in the knee. Some data from animal models suggest that damaged cartilage undergoes healing more efficiently when MSCs are injected into the injury, and this can be further enhanced if the MSCs are modified to produce growth factors associated with cartilage. It has been shown that once the MSCs are injected into the knee they attach themselves to the site of damage and begin to change into chondrocytes, promoting healing and repair. A small number of completed clinical trials in humans using MSCs to treat cartilage damage have reported some encouraging results, but these studies used very few patients, making it difficult to accurately interpret the results. There are currently a number of ongoing trials using larger groups of patients, and the hope is that these will provide more definite information about the role MSCs play in cartilage repair.
Tendinopathy

Tendinopathy relates to injuries that affect tendons – the long fibrous tissues that connect and transmit force from muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
MSCs have the ability to generate cells called tenoblasts that mature into tenocytes. These tenocytes are responsible for producing collagen in tendons. This link between MSCs and collagen is the focus for researchers investigating how stem cells may help treat tendinopathy. Substantial research has been carried out on racehorses as they suffer from high rates of tendinopathy, and the injury is similar to that found in humans. Researchers discovered that by injecting MSCs isolated from an injured horse’s own bone marrow into the damaged tendon, recurrence rates were almost cut in half compared to horses that receive traditional medical management for this type of injury. A later study by the same group showed the MSCs improved repair, resulting in reduced stiffness of the tissue, decreased scarring, and better fusion of the new fibers with the existing, undamaged tendon. It is not yet clear if these results are due to MSCs producing new tenocytes or their ability to modulate the environment around the tendinopathy, as described above. These promising results paved the way for the first pilot study in humans.
Bone Repair

Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially, there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone onto the cartilage. Finally, these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Brain Injury in Sports

There is mounting evidence that those taking part in sports where they are exposed to repetitive trauma to the head and brain are at a higher risk of developing neurodegenerative disorders, some of which are targets for stem cell treatments. For example, it has been reported that the rate of these diseases, like Alzheimer’s Disease, were almost four times higher in professional American football players compared to the general population. While the cause of this disease is not yet clear, it is associated with abnormal accumulation of proteins in neural cells that eventually undergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
Boxers suffering from dementia pugilistica, a disease thought to result from damage to nerve cells, can also demonstrate some symptoms of Parkinson’s Disease (among others). In healthy brains, specialized nerve cells called dopaminergic neurons produce dopamine, a chemical that transmits signals to the part of the brain responsible for movement. The characteristic tremor and rigidity associated with Parkinson’s Disease is due to the loss of these dopaminergic neurons and the resulting loss of dopamine production. Researchers are able to use stem cells to generate dopaminergic neurons in the lab that are used to study the development and pathology of this disease. While a recent study reported that dopaminergic neurons derived from human embryonic stem cells improved some symptoms of the disease in mice and rats, stem cell-based treatments are still in the development phase.
- Published in Blog
What’s the Skinny on Adipose Stem Cells Derived From Fat?
WEDNESDAY, 13 JANUARY 2016 / PUBLISHED IN BLOG
Introduction to Adipose Stem Cells
In this blog, I’ll share some of the results we’ve had using stem cell therapies in different ways to show you how you can utilize them in your office or clinic. Let’s start with stem cell treatments for cosmetic regenerative tissue enhancement. The procedure starts with taking fat from one location on the patient’s body and relocating it to the area you’re trying to enhance and combining that fat with a population of adipose (fat-derived) stem cells for best results.
The Science Behind Adipose Stem Cells
This theory, in part, was first published back in 2006 by Kotaro Yoshimura, M.D., Associate Professor, Department of Plastic Surgery at the University of Tokyo. Dr. Yoshimura demonstrated that stem cells harvested from fat are actually responsible for creating new adipocytes.
Does this mean fat is our friend? When it comes to therapeutic tissue treatments, it sure is! We used to believe that we had a set number of adipocytes and that these either grew or shrank depending on the amount of fat that our bodies were gaining or holding, but we now know better. Everyone has a population of stem cells that exist within their fat tissue that is responsible for replacing or replenishing mature adipocytes, and they’ll grow with weight gain. By attaching to fat tissue, those stem cells will actually help support expansion or weight gain.
Breast Augmentation and Skin Rejuvenation

Take, for instance, breast augmentation using this process. By taking fat from one location, relocating it, and adding stem cells with the fat to the breast tissue, you can reduce reabsorption of the fat tissue. In addition to being able to perform fat transfer for breast augmentation, you can also utilize the stem cells and platelet-rich plasma (PRP) when you need to rejuvenate the skin as well. One example would be a patient who had received an injection of stem cells plus platelet-rich plasma without a fat graft: in this case, the cells will be very angiogenic in nature, creating new blood vessels and generating a youthful glow. The cells can also help with collagen production so the patient gets smoother skin and help with scarring or the appearance of unevenness on the skin.
Adipose Stem Cells in Orthopedics
Adipose stem cells can also be utilized for regenerative results in orthopedics. A typical technique is to isolate the platelet-rich plasma from the peripheral blood and combine it with stem cells from the fat tissue. Our preference is to utilize the adipose stem cells, again, because of the massive volume of stem cells fat tissue delivers. We can obtain about five hundred times more mesenchymal type stem cells—stem cells that can differentiate into a variety of cell types—from adipose tissue than we can obtain from bone marrow. For this reason, in most cases we utilize the cells from the adipose tissue rather than the bone marrow.
Protocols and Success Stories in Orthopedics
This protocol comes courtesy of Joseph Purita, M.D., a member of the Global Stem Cells Group Advisory Board and a pioneer in the use of stem cells and PRP therapy for orthopedic surgery. Dr. Purita’s protocol is to inject the adipose cells plus the PRP intra-articularly to the affected joint.
This therapy has also been used successfully in animals. For instance, in the case of a horse with a chronic, non-healing tear in the ligament considered so chronic that they were going to put it down, an injection of the platelet-rich plasma plus the adipose stem cells directly into the lesion resulted in a complete resolution of the non-healing ligament within six months post-treatment.
Case Studies: Human Applications

Again, courtesy of Dr. Purita, is another example of a patient with avascular necrosis who had been told that she needed a total knee replacement. She was getting her knee drained once per week, had severe swelling and pain, and was not able to perform, pretty much, any activities due to her joint pain. After injection of the adipose cells plus the PRP, the patient was essentially pain-free, she was able to play tennis weekly, and there was complete resolution of the avascular necrosis, according to MRIs six months post-treatment.
Another example is a patient who was hit by a bus and thrown into a house, resulting in a non-union bone fracture that never healed properly. In this case, the patient was treated at the Hospital Angeles in collaboration with the Regenerative Medicine Institute. The patient had not been able to bear weight on the leg for more than two years. After an injection of stem cells plus a bone matrix, at the three-month follow-up, there was full continuity down the length of the bone, and for the first time in more than two years, the patient was able to bear weight.
Conclusion: The Future of Adipose Stem Cells
Treatments using adipose (fat)-derived stem cells, in combination with PRP and other regenerative medicine therapies, are proving to provide the body with the ability to heal in cases where nothing else worked. Initial findings tell us that PRP-assisted stem cells can figure out what cells they need to replicate—whether cartilage cells, bone cells, or collagen cells for ligaments and tendons—to help the body heal from within.
- Published in Blog
Supercharge your Regenerative Medicine Education


Global Stem Cells Group’s 2016 Regenerative Medicine Symposium
Global Stem Cells Group is proud to present our 2016 Edition of our Regenerative Medicine Symposium, to be held in 6 different Cities around the World. This prestigious event will have the presence of a select group of renowned international speakers who will offer a combined day of conferences of high scientific rigor aimed at Physicians.
Key Highlights of the Symposium
Global Stem Cells Group’s symposium will provide cutting-edge information on developments in all areas of stem cell research, including the biology, medicine, applications, regulations, product development, and the commercialization of stem cells.
Expert International Speakers
The symposium will feature a select group of renowned international speakers who will share their knowledge and expertise in stem cell research and applications. These experts will deliver high-level scientific presentations aimed at advancing the understanding and practice of regenerative medicine.
Comprehensive Coverage of Stem Cell Research
Attendees will gain insights into the latest advancements in stem cell biology, medical applications, regulatory landscapes, and product development. The symposium will cover a broad range of topics crucial for professionals in the field.
Business Opportunities in Stem Cell Research
Business opportunities, challenges, and potential strategies for overcoming these challenges will also be addressed. Attendees will learn about the current commercially viable categories of companies, funding strategies, and strategic relationships available within the industry.
Event Schedule and Locations
Our first event of the year will take place in Bogota, Colombia, on March 3rd. For more information and registration, click here.
- Published in Blog