(Almost) Everything You Wanted to Know About Stem Cells But Were Afraid to Ask
Discovering the Power of Stem Cells
Stem cells have captivated the interest of biologists, healthcare professionals, and the curious public alike for years. At Global Stem Cells Group, we are pioneering stem cell treatments for a wide range of medical conditions, making them accessible in physician offices and outpatient clinics worldwide, with plans to expand availability in the U.S. soon.
Understanding Stem Cells

A stem cell is defined by its unique ability to self-renew and differentiate into specialized cells needed for various treatments. These versatile cells can transform into muscle cells, heart cells, skin cells, and more through specific chemical and genetic signals manipulated by stem cell physicians.
Sources of Stem Cells

Different types of stem cells originate from various sources. Adult adipose-derived stem cells, found abundantly in fat tissue, are particularly favored for their ease of harvest and plentiful supply. Each liter of fat yields hundreds of millions of potential stem cells, capable of becoming fat, heart, bone, or muscle tissue.
Global Stem Cells Group’s Approach
Global Stem Cells Group offers validated, compliant outpatient methods for providing adipose-derived and bone marrow-derived stem cells to physicians worldwide. We have trained over 500 physicians who now offer stem cell therapies in their practices, pioneering the integration of regenerative medicine into mainstream healthcare.
How Stem Cell Therapy Works

Stem cell therapy leverages the body’s innate healing potential to reverse the effects of aging, degenerative diseases, and injuries. By guiding stem cells to replicate youthful and vigorous cells, we rejuvenate tissues and restore function. Stem cell therapy is evolving beyond regenerative medicine, combining techniques like gene therapy and advanced biologics for more targeted treatments.
The Future of Regenerative Medicine
Regenerative medicine represents a transformative shift in healthcare, akin to historical breakthroughs like the polio vaccine and antibiotics. Combining cellular and gene therapies promises new avenues for treating previously untreatable conditions, ushering in a new era of medical innovation and patient care.
- Published in Blog
Could stem cells repair the damaged brain in Alzheimer’s?
Exploring Stem Cell Therapies for Alzheimer’s Disease
Alzheimer’s disease presents a formidable challenge, characterized by progressive cognitive decline and neurological deterioration. At Global Stem Cells Group, we delve into the potential of stem cell therapies to revolutionize Alzheimer’s treatments, offering hope where conventional methods have fallen short.
Understanding Alzheimer’s Disease
Alzheimer’s primarily affects individuals aged 70 and older, with women more susceptible. This irreversible brain disorder impacts memory, cognition, and daily functioning, posing significant challenges for patients and caregivers alike.
Current Treatment Challenges
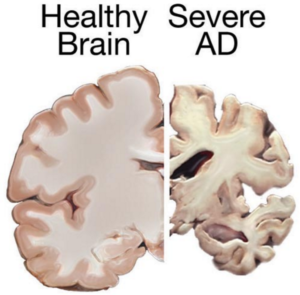
Despite extensive research, there is currently no cure for Alzheimer’s. Existing treatments focus on managing symptoms temporarily, offering limited relief from cognitive decline and behavioral changes.
The Role of Stem Cells in Alzheimer’s Treatment
Stem cell therapies offer a promising alternative by potentially replacing damaged neurons with healthy ones. Neural stem cells, for instance, could be transplanted into the brain to regenerate neurons and restore cognitive function. Challenges include ensuring cell integration and overcoming the presence of disease-causing proteins that may compromise long-term efficacy.
Potential Therapeutic Approaches
Mesenchymal Stem Cells in Alzheimer’s

Research suggests using stem cells to deliver neurotrophins, proteins crucial for neuron survival and function. Although promising results have been observed in animal studies, translating these findings to human treatments requires further exploration.
Mesenchymal stem cells show potential for their anti-inflammatory properties, which may mitigate Alzheimer’s symptoms. However, clinical studies are necessary to validate their safety and effectiveness in treating this complex neurodegenerative disorder.
Advancements in Stem Cell Research
Recent breakthroughs, such as reprogramming skin cells into induced pluripotent stem cells (iPSCs), offer new avenues for studying Alzheimer’s pathology and testing innovative treatments. These advancements provide hope for identifying novel therapeutic strategies.
Conclusion
While challenges persist in developing effective stem cell therapies for Alzheimer’s, ongoing research and clinical trials hold promise for advancing treatment options. Global Stem Cells Group remains committed to pioneering new approaches that could transform the landscape of Alzheimer’s care.
References
- http://www.eurostemcell.org/factsheet/alzheimer%E2%80%99s-disease-how-could-stem-cells-help
- http://hsci.harvard.edu/news/alzheimer%E2%80%99s-dish
- http://www.ipscell.com/2012/05/can-stem-cells-be-used-to-treat-alzheimers-disease/
- http://www.sciencedirect.com/science/article/pii/S1934590915003173
- http://www.cell.com/cell-stem-cell/abstract/S1934-5909%2815%2900305-7
- Published in Blog
Could stem cells offer the cure for muscular dystrophy?
Exploring Stem Cell Therapies for Muscular Dystrophy
Muscular dystrophy (MD) encompasses a group of genetic disorders causing progressive muscle weakness and loss of mass. At Global Stem Cells Group, we delve into the potential of stem cell treatments to revolutionize care for muscular dystrophy patients worldwide.
Understanding Muscular Dystrophy
Muscular dystrophy affects voluntary muscles, with symptoms ranging from mild to severe, impacting mobility and daily life. Duchenne muscular dystrophy (DMD), the most severe form, primarily affects young boys, leading to progressive muscle degeneration and shortened lifespan.
Challenges in Current Treatments
While treatments like physiotherapy and steroids aim to manage symptoms, there is no cure for DMD. These approaches often fall short due to side effects and limited effectiveness in halting disease progression.
The Promise of Stem Cells in Muscular Dystrophy
Stem cell therapies offer potential by addressing the underlying cause—defective dystrophin production. Research explores using stem cells from muscle tissue, bone marrow, and blood vessels to regenerate muscle fibers deficient in dystrophin, showcasing promising results in animal models.
Recent Advances and Research
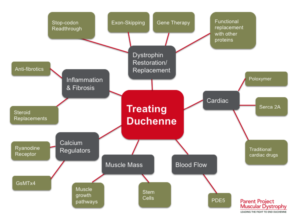
Studies have demonstrated that stem cells isolated from muscle blood vessels can restore mobility in affected animals. Researchers have also explored genetic correction combined with stem cell therapy, showing improvements in muscle regeneration and function in mice models of DMD.
Future Directions
While translating these findings to human treatments requires further research, combined therapies integrating stem cells and gene therapies show promise. These approaches aim to replace defective stem cells with healthy ones or reduce inflammation to preserve muscle function effectively.
Conclusion
While a cure for muscular dystrophy remains elusive, stem cell research holds immense promise. Global Stem Cells Group remains at the forefront, exploring innovative therapies that could transform the outlook for patients with muscular dystrophy.
References:
- http://www.mayoclinic.org/diseases-conditions/muscular-dystrophy/basics/definition/con-20021240
- http://www.eurostemcell.org/factsheet/muscular-dystrophy-how-could-stem-cells-help
- https://www.mda.org/disease/duchenne-muscular-dystrophy/research
- http://quest.mda.org/article/scientists-bullish-stem-cells-muscle-repair
- http://hsci.harvard.edu/stem-cells-used-treat-muscular-dystrophy-mice
- https://med.stanford.edu/news/all-news/2014/12/stem-cells-faulty-in-duchenne-muscular-dystrophy.html
http://www.mayoclinic.org/diseases-conditions/muscular-dystrophy/basics/definition/con-20021240
- http://www.eurostemcell.org/factsheet/muscular-dystrophy-how-could-stem-cells-help
- https://www.mda.org/disease/duchenne-muscular-dystrophy/research
- http://quest.mda.org/article/scientists-bullish-stem-cells-muscle-repair
- http://hsci.harvard.edu/stem-cells-used-treat-muscular-dystrophy-mice
- https://med.stanford.edu/news/all-news/2014/12/stem-cells-faulty-in-duchenne-muscular-dystrophy.html
- Published in Blog
Stem cells may be used for healing damaged lungs
Stem Cells: A Promising Approach for Lung Tissue Repair
Stem cell research offers new hope for treating debilitating lung conditions such as bronchitis, asthma, cystic fibrosis, and emphysema, which affect millions worldwide.
Research at the Weizmann Institute of Science
Scientists at the Weizmann Institute of Science have discovered that stem cells could potentially repair damaged lung tissues. Their study demonstrated that embryonic stem cells, similar to those found in bone marrow, can migrate to damaged lung compartments, differentiate, and regenerate lung tissue in mice models [1].
Implications of Lung-specific iPSCs
Previous studies, including research from the Boston University Medical Center, have focused on generating lung-specific induced pluripotent stem cells (iPSCs) from patients with lung diseases. These iPSCs show promise as they can be cultivated without embryos, reducing the risk of rejection in transplants. They have been found capable of differentiating into lung tissue precursor cells, offering a potential alternative to embryonic stem cells [2].
Future Directions in Stem Cell Therapy
Ongoing research aims to optimize stem cell transplantation methods for treating severe respiratory diseases. Scientists are exploring drug dosages to prevent rejection and considering the creation of stem cell banks to facilitate broader clinical applications.
Conclusion
While still in experimental stages, stem cell therapies represent a promising avenue for repairing damaged lungs and improving respiratory function. Continued research and development are crucial for translating these findings into effective treatments for patients worldwide.
References:
- Chava Rosen, Elias Shezen, Anna Aronovich, Yael Zlotnikov Klionsky et al. – Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice, Nature Medicine, 2015, http://www.nature.com/nm/journal/vaop/ncurrent/full/nm.3889.html
- Aba Somers, Jyh-Chang Jean, Cesar A. Sommer, Amel Omari et al. – Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette, Stem Cells, 2010, 28 (10):1728, http://onlinelibrary.wiley.com/doi/10.1002/stem.495/full
- Published in Blog
Adult tissues that serve as source for stem cells
Exploring Adult Stem Cells: Sources and Potential
Stem cells derived from adult tissues are gaining attention for their regenerative potential across various medical conditions, offering hope for treating diseases like osteoarthritis, muscular dystrophy, and Alzheimer’s.
Bone Marrow: A Rich Source of Stem Cells
Bone marrow harbors hematopoietic stem cells (HSCs) capable of differentiating into various blood cell types—essential for immune function and blood clotting. Additionally, skeletal stem cells (STCs) contribute to bone and cartilage regeneration [1].
Neural Stem Cells (NSCs) in Brain Research
Neural stem cells found in the brain show promise in replacing dying neurons, highlighting potential applications in neurological disorders such as multiple sclerosis and Parkinson’s disease [2].
Intestinal Stem Cells (ISCs) and Their Role
ISCs, lining the intestines, continuously renew tissue throughout life but pose challenges in cancer contexts where they can contribute to tumor regeneration post-treatment.
Liver: A Hub of Regeneration
Hepatocytes, liver’s primary cells, exhibit stem cell-like properties, crucial for regenerating liver tissue damaged by infections or surgery. Research suggests specialized stem cells from the biliary tree could aid in diabetes treatment by generating insulin-producing islets [3].
Future Directions and Conclusion
Research into adult stem cells from diverse tissues offers insights into their therapeutic potential, bypassing ethical concerns associated with embryonic stem cells. Lower rejection risks and higher differentiation rates make them promising candidates for future regenerative medicine applications.
REFERENCES:
[1] MacKlis, Jeffrey D.; Magavi, Sanjay S.; Leavitt, Blair R. (2000). “Induction of neurogenesis in the neocortex of adult mice”. Nature 405 (6789): 951–5[2] Biliary Tree Stem Cells, Precursors to Pancreatic Committed Progenitors: Evidence for Possible Life-long Pancreatic Organogenesis – http://www.diabetesresearch.org/file/research-publications/2013-Stem-Cells_Biliary-Tree-Stem-Cells-to-Islets.pdf
- Published in Blog
A Good Night’s Sleep Protects Stem Cells From Premature Aging
Understanding the Role of Sleep in Stem Cell Health
Research reveals that adequate sleep not only rejuvenates the body but also safeguards adult hematopoietic stem cells from premature aging and DNA damage, contributing to overall health and longevity.
Impact of Sleep on Stem Cell Dormancy
German Cancer Research Center scientists have highlighted that adult stem cells remain dormant under normal conditions, protecting them from DNA damage. Sleep plays a crucial role in maintaining this dormancy, thereby preserving stem cell integrity and preventing premature aging [1].
Stress and Stem Cell Activity
Increased stress levels, whether from chronic infections or environmental factors, prompt stem cells to rapidly divide and activate. This heightened activity increases metabolic rates and DNA synthesis, elevating the risk of DNA damage and accelerating tissue aging [1, 3].
Link Between Stem Cells, Sleep, and Cancer Risk
Stress-induced damage in stem cells can lead to the accumulation of reactive metabolites that damage DNA, potentially increasing susceptibility to cancer. Understanding these mechanisms could aid in developing strategies to mitigate aging and cancer risks [1].
Circadian Rhythms and Skin Stem Cells
Studies from the University of California Irvine underscore the role of circadian rhythms in regulating skin stem cell metabolism. Adequate sleep supports healthy cell division and differentiation, crucial for maintaining skin health and repair processes [2].
Potential Implications for Human Health
While studies often begin with animal models like mice, the findings emphasize the importance of sleep in regulating stem cell function. Disruptions to circadian rhythms may compromise stem cell metabolism, leading to accelerated aging and potential health implications [2].
Conclusion
Research into the connection between sleep, stem cell health, and aging underscores the importance of maintaining healthy sleep patterns. These insights may pave the way for novel interventions aimed at preserving stem cell function and promoting overall health.
References:
[1] http://www.sciencedaily.com/releases/2015/02/150218122951.htm[2] http://www.cell.com/cell-reports/abstract/S2211-1247%2814%2901018-3
[3] http://en.wikipedia.org/wiki/DNA_damage_theory_of_aging
- Published in Blog